Contents of Article
- Summary
- What is Bioelectrical Impedance Analysis?
- Types of Bioelectrical Impedance Analysis
- What are the Bioelectrical Impedance Analysis equations?
- Is Bioelectrical Impedance Analysis valid and reliable?
- Are there issues with Bioelectrical Impedance Analysis?
- Is future research needed with Bioelectrical Impedance Analysis?
- Conclusion
- References
- About the Author
Summary
Bioelectrical Impedance Analysis (BIA) is able to make an estimation of body composition (e.g. quantities of fat mass and fat-free mass) by running a small electrical current through the body. This is possible simply because different bodily tissues (e.g. muscle, fat, bone, etc.) all have varying amounts of water content, and, as a result, they all differ in terms of electrical conductivity.
Despite being popular in many commercial gyms and within epidemiological research on group body composition, BIA does not appear to provide valid single- or repeated-measures of body composition for athletes. Having said that, the development of an equation for athletic populations that are validated against the gold-standard four-compartment model may improve the validity of the measure.

What is Bioelectrical Impedance Analysis?
First commercially available in the mid-1980s [1], Bioelectrical Impedance Analysis (BIA) is an inexpensive and portable piece of body composition testing equipment. Although BIA was primarily used to determine changes in dialysis patients [2], it is a method now used to determine body composition across a range of populations, including athletes [2, 3], obese individuals [4, 5], and the general population [3].
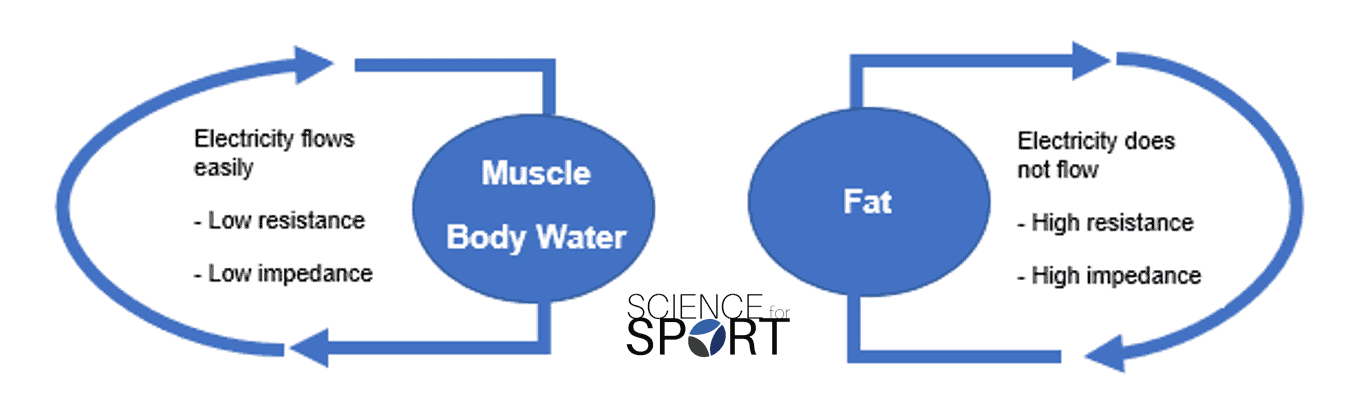
BIA determines body composition by running small electrical currents through the body. As the electrical conductivity is different between various bodily tissues (e.g. muscle, fat, bone, etc.) due to their variation in water content, the small electrical current passes through the tissues at different speeds. Armed with that information, the machine is able to calculate the impedance (i.e. the resistance of the electrical current [Z]) of the current and then estimate body composition – hence the name “bioelectrical impedance”.
The principle of BIA is that the different tissues in the body will act as conductors, semiconductors, or dielectrics (insulators). Lean tissues are highly-conductive, as they contain large quantities of water. In contrast, bone and adipose tissue are dielectric substances and are poor conductors [4]. BIA assumes that the human body is composed of a series of cylinders, uniform in shape, length, cross-sectional area, and with constant conductivity.
Total body water (TBW) is estimated, and this estimation is used to calculate fat-free mass. This is done under the assumption that 73 % of the body’s fat-free mass is water and that this remains constant over time and between individuals. Fat mass is then calculated as the difference between fat-free mass and body mass.
Types of Bioelectrical Impedance Analysis
Several methods have been used to assess body composition in humans, each with advantages and drawbacks surrounding cost, validity, reliability, and accessibility.
Bioelectrical impedance methods are often classified by the number of frequencies used for analysis, with BIA often referring to single-frequency devices, whereas multiple-frequency devices are referred to as ‘Bioelectrical Spectroscopy’ (BIS) as it uses a ‘spectra’ of frequencies [5]. It is unclear how many frequencies would be needed for a BIA device to be considered a BIS device, however, the principles behind how the devices work are the same. Therefore, for this review, BIA will be used to denote all bioelectrical impedance assessments.
Hand-held BIA
Different types of BIA analysers are available, such as hand-held and leg-to-leg devices. Hand-held BIA machines assess the conductance of a small alternating current through the upper body and use built-in software to calculate body composition after it has been calibrated with the following variables: weight, height, age, and gender [6]. This method may be of benefit in a field setting, due to its convenience.
Leg-to-Leg BIA
Similar to hand-held methods, leg-to-leg BIA involves an individual standing on scales with four electrodes situated at each footplate, with a low-level current passed through the lower body. The path of the electrical current may differ between this method and the hand-held method, and could potentially influence body composition results; though this issue is discussed later in the article.
Hand-to-Foot BIA
Hand-to-foot BIA uses electrodes in a mounted footplate, as well as electrodes in hand grips, to determine whole-body measurements. As hand-held and leg-to-leg methods may not account for the resistance of the lower- or upper body, respectively, it is logical to assume that hand-to-foot measurements may better reflect whole-body composition than the alternatives.
What are the Bioelectrical Impedance Analysis equations?
Estimates of body composition using BIA are facilitated using empirically validated equations, which consider variables including gender, race, height, weight, and age. Consequently, it is important the correct equation is used for the population measured to ensure that any results are valid. It is also important to understand the reference assessment method used to validate these equations.
For example, many BIA equations are validated against assessment methods such as hydrostatic weighing and Dual-energy X-ray Absorptiometry (DEXA). However, these methods can also lead to error, as hydrostatic weighing has been shown to lead to individual error rates of 6 % [6]. From the results of this assessment method, the manufacturer constructs an equation using the individual variables mentioned previously to determine what the body fat would be.
These equations will have an error rate when compared to the hydrostatic weighing method, and thus, this error is multiplied by the original error of the reference method to provide a body composition assessment that may be somewhat distant from the actual values reported using a four-compartment model.
Is Bioelectrical Impedance Analysis valid and reliable?
The validity (the agreement between the true value and a measurement value) of body composition is key to determining the precision of BIA measurement, and its suitability for clinical use. The criterion method for determining body composition is the four-compartment model (1] fat mass, 2] total body water, 3] bone mineral mass, and 4] residual mass), and should be used when assessing the validity of BIA measurements.
BIA has been compared to the four-compartment model in several studies using various populations. Sun et al., [7] validated BIA equations using the four-compartment model and reported that the equation was sufficient for use in epidemiological research studies to assess populations with normal levels of body composition.
Sun et al. (2003) [8] stated that BIA is a suitable alternative for estimating body fat percentages when subjects are within a “normal” body fat range, however, there is a tendency for BIA to overestimate body fat in lean subjects and underestimate body fat in obese individuals. It is important to note that this analysis utilised DEXA as the reference method, which may also lead to further error, as eluded to earlier in this review (read my article on the use of DEXA scanning for body composition assessment HERE).
The validity of BIA for one-off measures of body composition
Despite studies showing promising effects of BIA on body composition, this has not been found in a large body of research. BIA has been shown to underestimate fat mass and overestimate fat-free mass by 1.9 and 1.8 kg in obese subjects, respectively [9]. This finding is supported by other research on bodybuilders, showing that BIA underestimated fat mass, and overestimated fat-free mass when compared to the four-compartment model [10]. Research conducted by Jebb et al. (2000) [11] found that leg-to-leg BIA using the manufacturer equations resulted in large errors when attempting to predict body fat, relative to the four-compartment model. The authors subsequently developed a novel prediction equation to estimate fat mass from the same Tanita bioimpedance analyser, with the four-compartment method as a reference. However, later research found that this equation also failed to outperform the Tanita manufacturer equation, and resulted in wide limits of agreement [12].
Potentially of greater concern to practitioners considering the use of BIA to determine body composition in the applied setting, are the individual error rates of BIA, rather than data on group means. The study mentioned previously on obese subjects [9] reported that in 12 of the 50 participants, BIA underestimated fat mass by 5 kg or more. This is supported by the findings of Van Marken Lichtenbelt et al., [10], who reported an 8 % individual error rate when comparing BIA with the four-compartment model. This suggests that BIA may provide data that is not sufficiently accurate for the determination of individual body composition.
The validity of using BIA to measure changes over time
A further consideration for the use of BIA is the validity of its use in measuring changes in fat mass and fat-free mass over time, as this may indicate the efficacy of a nutritional or training intervention looking to manipulate body composition. To revisit the study by Ritz et al. (2007) [9], BIA was unable to accurately assess changes in body composition when compared to the four-compartment model. Fat mass was underestimated by 1.6 kg, whereas fat-free mass was overestimated by 1.8 kg. Individual error rates were greater than at baseline, with BIA underestimating fat mass by 7.5 kg in some subjects.
A further study on obese populations [13] showed individual disagreement in body fat measurement between BIA and the four-compartment model was high. Individual measures of body fat ranged from -3.6 % to 4.8 % of the four-compartment value, highlighting the potential for significant discrepancies when measuring individual body composition over time. BIA is likely to misrepresent changes over time, potentially missing significant changes in body composition, or suggesting changes that haven’t occurred.
There are a limited amount of comparisons between BIA and the reference four-compartment model in athletic populations. There is disagreement amongst the limited research available, with only one study suggesting that BIA is suitable for assessing body composition in athletes [15], whereas other research suggests that body fat estimates are much higher in athletes when using the BIA method [16].
The discrepancies between the studies may be due to various issues including differences in methodology, equations, and athletic population. There are currently no BIA equations for athletes that have been derived from the criterion four-compartment method (fat mass, total body water, bone mineral mass, residual mass). This makes the application of BIA in this population difficult, as athletes are likely to possess substantially different quantities of fat and fat-free mass when compared to the general population or diseased populations that current equations are based on.
The reliability of BIA
The reliability of BIA (the reproducibility of the observed value when the measurement is repeated) is also important to determine single-measurement precision, as well as the ability to track changes over time. A plethora of research has indicated the importance – and potentially the inability – of standardising BIA measures to sufficiently account for various confounders.
The mean coefficient of variation for within-day, intra-individual measurements, has ranged from 0.3 % to 2.8 %, with daily or weekly variability ranging from 0.9 % to 3.6 %, respectively [2, 17]. Standard measurement conditions may vary depending on the machine type (e.g. hand-to-hand, leg-to-leg, supine vs. standing, etc.). Other factors which may impact the BIA measurement and should therefore also be standardised are [16]:
- Room temperature
- Placement of electrodes
- Preparation of the skin
- Hydration status
- The analyser itself
The standardisation of hydration status is clearly of importance for BIA, as the method is reliant on estimations of total body water to ascertain fat-free mass. For female athletes, difference in hydration status during menses may significantly alter impedance [17] and should be a consideration when assessing female athletes with BIA.
Saunders et al. (1998) [18] showed that BIA was not a suitable method of body composition assessment in athletes with abnormal hydration status (e.g. hyperhydrated or hypohydrated), indicating that even small changes in fluid balance that occur with endurance training may be interpreted as a change in body fat content.

In addition, eating and strenuous exercise 2-4 hours prior to assessment have also previously been shown to decrease impedance; ultimately affecting the accuracy of the measurement [19]. The need to standardise eating, exercise, and both acute and chronic hydration changes are clearly important to provide valid body composition estimations.
Are there issues with Bioelectrical Impedance Analysis?
As mentioned previously, there are several issues with BIA measurement that may limit its use in an applied setting. Methodological limitations of BIA may affect the ability of the method to accurately determine body composition. The primary issues with BIA are:
- Sensor Placement
- Hydration and Glycogen Levels
- Effect of incorrect measures in the applied setting
- Variations in manufacturers’ equations
Sensor Placement
One such limitation is the placement of the sensors, and their ability to give readings of total body composition. As electrical current follows the path of least resistance, some scales may send current through the lower body only, missing the upper body entirely. Similarly, hand-held instruments may only assess the body composition of the upper extremities.
As females typically have a higher proportion of adipose tissue in the gluteal-femoral region [20], it is possible that this would not be represented using hand-held BIA devices. Hand-to-foot BIA devices, however, may allow for greater accuracy, as the current is sent from the upper body to the lower body, and is less likely to be influenced by the distribution of body fat.
Hydration and Glycogen Levels
Regardless, all devices are still subject to the same limitations that other BIA devices are. The assumption that the hydration fraction of skeletal muscle remains at 73 % is based on the chemical analysis of six cadavers as part of the Brussels Cadaver Analysis Study [21]. However, hydration of fat-free mass has been shown to rise to over 77 % with increased levels of body fat [22].
Deurenberg et al. (1988) [23] reported an underestimation of fat-free mass when assessing changes in body composition following weight loss. They speculated that changes in glycogen stores, and the loss of water bound to glycogen molecules, may affect BIA estimates of fat-free mass. In athletic populations, where varying glycogen stores are likely throughout a training week, it is likely that this will lead to some variation in the detection of change in fat-free mass in athletes as glycogen is likely to be affected by both diet, as well as the intensity, duration, and modality of previous training sessions – even with protocol standardisation.
Effect of incorrect measures in the applied setting
An important consideration when assessing the individual variation of BIA is the potential consequences that an incorrect reading can have. As reported earlier, error rates can range from 4-8 % for individuals, and, therefore, an athlete could have decreased body fat percentage by 4 %, whereas their BIA would report that they had gained 4 % body fat. This can have wide-ranging implications, from assessing the efficacy of previous dietary and training interventions to making decisions on the correct interventions moving forward.
For example, an athlete may be singled out for interventions to reduce their body fat based on their BIA assessment and normative values, yet other methods may suggest that their body composition is optimal. Without considered interpretation of this result, coaches may question an athlete’s commitment and professionalism if they believe that their body fat has increased drastically over time. Similarly, athletes often take interest in their body fat percentages, and a false score indicating so-called “negative” changes in body fat may impact the confidence and compliance of an athlete.
Is future research needed with Bioelectrical Impedance Analysis?
The primary area for future research in this area is clearly the need for validated BIA equations for athletes in a range of sports and with varying body composition. It is important that these equations are validated using a total-body, water-based, four-compartment method, in an attempt to minimise the measurement error that is found when equations are based on the two-compartment model; such as hydrostatic weighing. As such, the following areas of research are needed to expand current knowledge on this topic:
- A BIA equation for use in athletic populations
- The validity of BIA vs. the four-compartment model for athletes with “more extreme” body composition (e.g. greater muscle mass, leaner, etc.)
- The ability of BIA to accurately detect changes in athletes’ body composition over a period of time
Conclusion
To conclude, it is likely that BIA is not a suitable body composition assessment method for athletic populations. The lack of a validated equation for this population, combined with the large individual error reported in overweight and obese populations, suggests that BIA does not provide accurate body composition data for both single-measure and repeated measures.
- Buccholz, C. Bartok and D. A. Schoeller, “The Validity of Bioelectrical Impedance Models in Clinical Populations,” Nutrition in Clinical Practice, vol. 19, no. 5, pp. 443-446, 2004. https://www.ncbi.nlm.nih.gov/pubmed/16215137
- Nyboer and J. A. Sedensky, “Bioelectrical impedance during renal dialysis,” Nephrology Dialysis Transportation, vol. 4, pp. 214-219, 1974. https://www.ncbi.nlm.nih.gov/pubmed/4468420
- M. E. Franssen, E. P. A. Rutten, M. T. J. Groenen, L. E. Vanfleteren, E. F. M. Wouters and M. A. Spruit, “New reference values for body composition by bioelectrical impedance analysis in the general population: Results from the UK Biobank,” Journal of the American Medical Directors Association, vol. 15, no. 6, pp. 1-6, 2014. https://www.ncbi.nlm.nih.gov/pubmed/24755478
- Scharfetter, T. Schlager, R. Stollberger, R. Felsberger, H. Hutten and H. Hinghofer-Szalkav, “Assessing abdominal fatness with local bioimpedance analysis: basics and experimental findings,” International Journal of Obesity and Related Metabolic Disorders, vol. 25, no. 4, pp. 502-511, 2001. https://www.ncbi.nlm.nih.gov/pubmed/11319654
- R. Moon, “Body composition in athletes and sports nutrition: an examination of the bioimpedance analysis technique,” European Journal of Clinical Nutrition, vol. 67, pp. 54-59, 2013. https://www.ncbi.nlm.nih.gov/pubmed/23299872
- A. Bergsma-Kadijk, B. Baumeister and P. Deurenberg, “Measurement of body fat in young and elderly women: comparison between a four-compartment model and widely used reference methods.,” British Journal of Nutrition, vol. 75, no. 5, pp. 649-657, 1996. https://www.ncbi.nlm.nih.gov/pubmed/8695593
- S. Sun, C. W. Chumlea, S. B. Heymsfield , H. C. Lukaski, D. Schoeller, K. Friedl, R. J. Kuczmarski, K. M. Flegal, C. L. Johnson and V. S. Hubbard, “Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys,” American Journal of Clinical Nutrition, vol. 77, pp. 331-340, 2003. https://www.ncbi.nlm.nih.gov/pubmed/12540391
- Sun, C. R. French, G. R. Martin, B. Younghusband, R. C. Green, Y. Xie, M. Matthews, J. R. Barron, D. G. Fitzpatrick, W. Gulliver and H. Zhang, “Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population,” American Journal of Clinical Nutrition, vol. 81, pp. 74-78, 2005. https://www.ncbi.nlm.nih.gov/pubmed/15640463
- Ritz, A. Salle, M. Audran and V. Rohmer, “Comparison of different methods to assess body composition of weight loss in obese and diabetic patients,” Diabetes Research and Clinical Practice, vol. 77, pp. 405-411, 2007. https://www.sciencedirect.com/science/article/pii/S0168822707000332
- D. van Marken Lichtenbelt, F. Hartgens, N. B. Vollaard, S. Ebbing and H. Kuipers, “Body composition changes in bodybuilders: a method comparison,” Medicine and Science in Sport and Exercise, vol. 36, no. 3, pp. 490-497, 2004. https://www.ncbi.nlm.nih.gov/pubmed/15076792
- A. Jebb, T. J. Cole, D. Doman, P. R. Murgatroyd and A. M. Prentice, “Evaluation of the novel Tanita body-fat analyser to measure body composition by comparison with a four-compartment model,” British Journal of Nutrition, vol. 83, pp. 115-122, 2000. https://www.ncbi.nlm.nih.gov/pubmed/10743490
- E. Chouinard, D. A. Schoeller, A. C. Watras, R. Randall Clark, R. N. Close and A. C. Buchholz, “Bioelectrical Impedance vs. Four-compartment Model to Assess Body Fat Change in Overweight Adults,” Obesity, vol. 15, pp. 85-92, 2007. https://www.ncbi.nlm.nih.gov/pubmed/17228035
- M. Evans, M. J. Saunders, M. A. Spano, S. A. Arngrimsson, R. D. Lewis and K. J. Cureton, “Body-composition changes with diet and exercise in obese women: A comparison of estimates from clinical methods and a 4-component model,” American Journal of Clinical Nutrition, vol. 70, pp. 5-12, 1999. https://www.ncbi.nlm.nih.gov/pubmed/10393132
- Andreoli, G. Melchiorri, S. L. Volpe and A. De Lorenzo, “Multicompartment model to assess body composition in professional water polo players,” Journal of Sports Medicine and Physical Fitness, vol. 44, pp. 38-43, 2004. https://www.ncbi.nlm.nih.gov/pubmed/15181388
- R. Clark, C. Bartok, J. C. Sullivan and D. A. Schoeller, “Minimum Weight Prediction Methods CrossValidated,” Medicine and Science in Sport and Exercise Science, vol. 36, no. 4, pp. 639-647, 2004. http://europepmc.org/abstract/med/15064592
- R. F, “Bioelectrical Impedance Analysis: A review of principles and applications,” Journal of the American College of Nutrition, vol. 11, no. 2, pp. 199-209, 1992. https://www.ncbi.nlm.nih.gov/pubmed/1578098
- N. Gleichauf and D. A. Roe, “The menstrual cycle’s effect on the reliability of bioimpedance measurements for assessing body composition.,” American Journal of Clinical Nutrition, vol. 50, pp. 903-907, 1989. https://www.ncbi.nlm.nih.gov/pubmed/2816797
- J. Saunders, J. E. Blevins and C. E. Broeder, “Effects of hydration changes on bioelectrical impedance in endurance trained individuals,” Medicine and Science in Sport and Exercise, vol. 30, pp. 885-892, 1998. https://www.ncbi.nlm.nih.gov/pubmed/9624647
- Deurenberg, J. A. Weststrate, I. Paymans and K. Van Der Kooy, “Factors affecting bioelectical impedance measurements in humans,” European Journal of Clinical Nutrition, vol. 42, pp. 1017-1022, 1988. https://www.ncbi.nlm.nih.gov/pubmed/9624647
- Lemieux, D. Prud’homme, C. Bouchard, A. Tremblay and J. P. Despres, “Sex differences in the relation of visceral adipose tissue accumulation to total body fatness.,” American Journal of Clinical Nutrition, vol. 58, no. 4, pp. 463-467, 1993. https://www.ncbi.nlm.nih.gov/pubmed/8379501
- P. Clarys, A. D. Martin and D. T. Drinkwater, “Gross tissue masses in adult humans- data from 25 dissections,” Human Biology, vol. 56, pp. 459-473, 1984. https://www.researchgate.net/publication/16706298_Gross_Tissue_Weights_in_the_Human_Body_by_Cadaver_Dissection
- R. Segal, J. Wang, B. Gutin, R. N. Pierson and T. Van Italie, “Hydration and potassium content of lean body mass: effects of body fat, sex and age,” American Journal of Clinical Nutrition, vol. 45, p. 865, 1987. https://www.sciencedirect.com/science/article/pii/002196818290056X
- Deurenberg, J. A. Weststrate and J. G. A. J. Hautvast, “Changes in fat-free mass during weight loss measured by bioelectrical impedance and by densitometry,” American Journal of Clinical Nutrition, vol. 49, pp. 33-36, 1989. https://www.ncbi.nlm.nih.gov/pubmed/2912008
- C. Lukaski, W. W. Bolonchuk, W. A. Siders and C. B. Hall, “Body composition assessment of athletes using bioelectrical impedance measurements,” Journal of Sports Medicine and Physical Fitness, pp. 434-440, 1990. https://www.ncbi.nlm.nih.gov/pubmed/2079851
- R. Esco, M. S. Olson, H. N. Williford, S. N. Lizana and A. R. Russell, “The accuracy of hand to hand bioelectrical impedance analysis in predicting body composition in college-age female athletes,” Journal of Strength and Conditioning Research, vol. 25, no. 4, pp. 1040-1045, 2011. https://www.ncbi.nlm.nih.gov/pubmed/20647951
- F. Kushner, R. Gudivaka and D. A. Schoeller, “Clinical characteristics influencing bioelectrical impedance analysis measurements,” American Journal of Clinical Nutrition, vol. 64, pp. 423-427, 1996. https://www.ncbi.nlm.nih.gov/pubmed/8780358
- M. W. J. Schols, A. M. C. Dingemans, P. B. Soeters and E. F. M. Wounters, “Within-day variation of bioelectrical resistance measurements in patients with chronic obstructive pulmonary disease,” Clinical Nutrition, vol. 9, pp. 266-271, 1990. https://www.ncbi.nlm.nih.gov/pubmed/16837369
- F. Kushner and D. A. Schoeller, “Estimation of total body water in bioelectrical impedance analysis,” American Journal of Clinical Nutrition, vol. 44, pp. 417-424, 1986. https://www.ncbi.nlm.nih.gov/pubmed/3529918